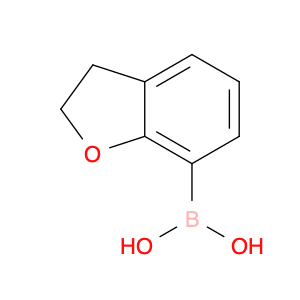
The (2,3-Dihydrobenzofuran-7-yl)boronic acid is a crucial reagent in chemical synthesis, particularly in the field of organic chemistry. This compound is widely used in various synthetic transformations due to its versatility and reactivity. In organic synthesis, (2,3-Dihydrobenzofuran-7-yl)boronic acid serves as a key building block for the construction of complex molecules, such as pharmaceuticals, agrochemicals, and materials.One of the primary applications of (2,3-Dihydrobenzofuran-7-yl)boronic acid is in Suzuki-Miyaura cross-coupling reactions. This palladium-catalyzed coupling reaction allows for the formation of carbon-carbon bonds between an aryl or vinyl boronic acid and an organic halide or pseudohalide. By utilizing (2,3-Dihydrobenzofuran-7-yl)boronic acid as a boronic acid partner in this reaction, chemists can introduce the (2,3-Dihydrobenzofuran-7-yl) group into a target molecule selectively and efficiently.Additionally, (2,3-Dihydrobenzofuran-7-yl)boronic acid can participate in other types of organic transformations, such as palladium-catalyzed borylation reactions, Heck couplings, and C-H activation reactions. Its ability to serve as a synthetically valuable intermediate in these reactions makes it a valuable tool for chemists looking to access novel molecular structures and compounds.Overall, the application of (2,3-Dihydrobenzofuran-7-yl)boronic acid in chemical synthesis offers a powerful strategy for the construction of complex molecules and the diversification of chemical libraries for various applications in the fields of pharmaceuticals, materials science, and agrochemicals.
 sales@aaronchem.com
sales@aaronchem.com