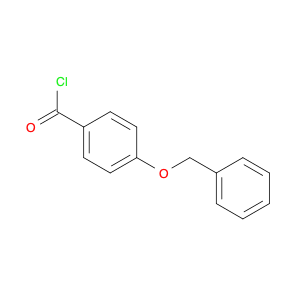
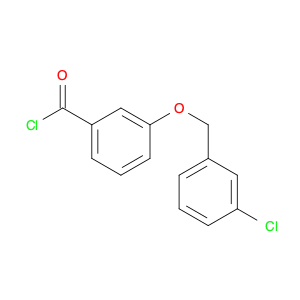
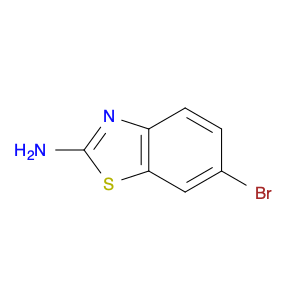
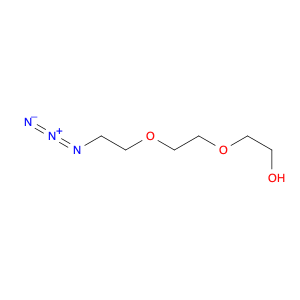
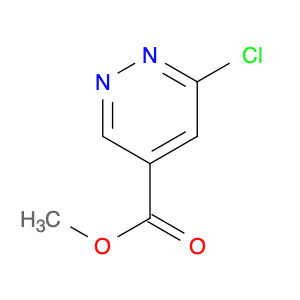
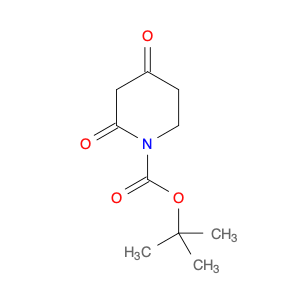
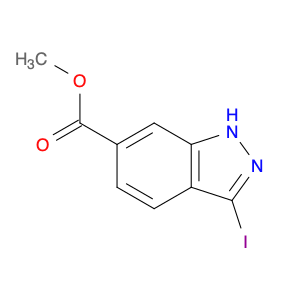
3-BENZYLOXY-BENZOYL CHLORIDE is a versatile compound widely used in chemical synthesis as a key intermediate in the preparation of various pharmaceuticals, agrochemicals, and specialty chemicals. It serves as a valuable building block for the synthesis of complex organic molecules due to its unique reactivity and structural properties.One common application of 3-BENZYLOXY-BENZOYL CHLORIDE is in the synthesis of benzophenone derivatives, which have a wide range of industrial and pharmaceutical applications. By reacting with nucleophiles such as amines, alcohols, or thiols, 3-BENZYLOXY-BENZOYL CHLORIDE can undergo nucleophilic substitution reactions to introduce functional groups at specific positions on the benzophenone scaffold.Additionally, 3-BENZYLOXY-BENZOYL CHLORIDE is often utilized in the preparation of esters and amides through acylation reactions. By reacting with various alcohols or amines in the presence of a base or acid catalyst, this compound can facilitate the formation of ester or amide bonds, allowing for the synthesis of diverse chemical compounds with tailored properties.Furthermore, 3-BENZYLOXY-BENZOYL CHLORIDE can also participate in cross-coupling reactions with organometallic reagents, such as Grignard reagents or organolithium compounds, to form biaryl compounds or other complex organic structures. These cross-coupling reactions enable the efficient construction of carbon-carbon bonds, crucial for the synthesis of intricate molecular architectures.Overall, the versatile reactivity and functional group compatibility of 3-BENZYLOXY-BENZOYL CHLORIDE make it a valuable reagent in chemical synthesis, enabling the efficient and controlled assembly of diverse organic compounds with potential applications in various industrial sectors.
 sales@aaronchem.com
sales@aaronchem.com