Login |
Create New Account
 sales@aaronchem.com
sales@aaronchem.com
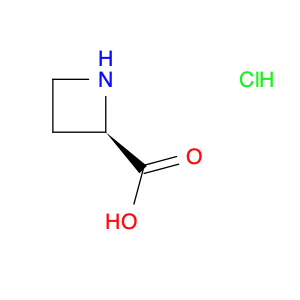
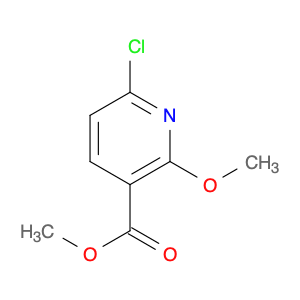
(S)-(-)-2-Azetidinecarboxylic acid
Catalog#: AR0038AJ | CAS#: 2133-34-8 | MDL#: MFCD00005166 | MF: C4H7NO2 | MW: 101.1039
| Packsize | Purity | Price | Availability | Quantity | ||
|---|---|---|---|---|---|---|
| 50mg | $5.00 | Global Stock | Buy Now | Add To Cart | ||
| 100mg | $8.00 | Global Stock | Buy Now | Add To Cart | ||
| 250mg | $9.00 | Global Stock | Buy Now | Add To Cart | ||
| 1g | $20.00 | Global Stock | Buy Now | Add To Cart | ||
| 5g | $57.00 | Global Stock | Buy Now | Add To Cart | ||
| 10g | $111.00 | Global Stock | Buy Now | Add To Cart | ||
| 25g | $275.00 | Global Stock | Buy Now | Add To Cart | ||
| 100g | $1,077.00 | Global Stock | Buy Now | Add To Cart |
- Description
- Application
- Related Products
- Featured Products
| Catalog Number | AR0038AJ |
| Chemical Name | (S)-(-)-2-Azetidinecarboxylic acid |
| CAS Number | 2133-34-8 |
| Molecular Formula | C4H7NO2 |
| Molecular Weight | 101.1039 |
| MDL Number | MFCD00005166 |
| SMILES | OC(=O)[C@@H]1CCN1 |
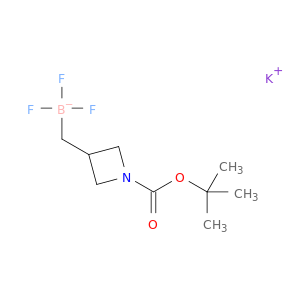
Potassium ((1-[(tert-butoxy)carbonyl]azetidin-3-yl)methyl)trifluoroboranuide
2254447-10-2