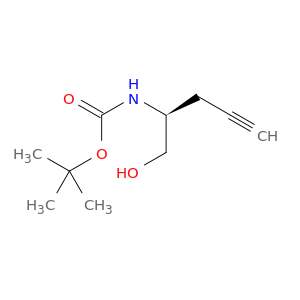
(S)-tert-Butyl (1-hydroxypent-4-yn-2-yl)carbamate, commonly known as $name$, plays a crucial role in chemical synthesis as a versatile building block. This compound is a key intermediate in the preparation of various organic compounds due to its unique chemical properties. One of the main applications of $name$ is in the synthesis of complex molecules, particularly in the formation of heterocycles and bioactive compounds.In organic synthesis, (S)-tert-Butyl (1-hydroxypent-4-yn-2-yl)carbamate is utilized as a chiral auxiliary, enabling the creation of enantioselective products. Its chirality imparts stereochemical control to reactions, leading to the formation of specific enantiomers with high selectivity. This compound is particularly valuable in the preparation of pharmaceuticals, agrochemicals, and other fine chemicals where stereochemistry is crucial for biological activity or functionality.Furthermore, (S)-tert-Butyl (1-hydroxypent-4-yn-2-yl)carbamate acts as a protecting group for amines, allowing selective deprotection under mild conditions. This protective strategy is essential in multi-step synthesis to prevent undesired reactions and facilitate the isolation of pure products. By strategically incorporating and removing this protecting group, chemists can efficiently construct intricate molecular structures with precision.Overall, the versatile applications of (S)-tert-Butyl (1-hydroxypent-4-yn-2-yl)carbamate make it an indispensable tool in the toolkit of synthetic chemists, enabling the efficient and selective synthesis of diverse organic compounds with tailored stereochemistry and functionality.
 sales@aaronchem.com
sales@aaronchem.com