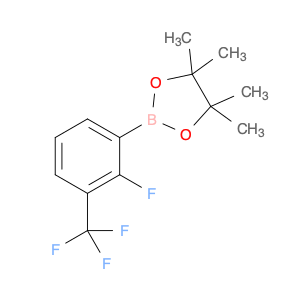
The compound 2-[2-Fluoro-3-(trifluoromethyl)phenyl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane is a valuable boron-containing building block commonly used in chemical synthesis. This compound is utilized in various organic reactions, particularly in the field of transition metal-catalyzed cross-coupling reactions. Its unique structure featuring a boron atom and fluorinated phenyl groups makes it a versatile reagent in the formation of carbon-carbon bonds.In chemical synthesis, 2-[2-Fluoro-3-(trifluoromethyl)phenyl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane serves as a boron source that can be easily functionalized to introduce specific functional groups into organic molecules. It is commonly employed in Suzuki-Miyaura cross-coupling reactions, where it reacts with aryl or vinyl halides in the presence of a palladium catalyst to form biaryl or styrenyl compounds. This reaction is widely used in pharmaceutical, agrochemical, and material science research for the construction of complex organic molecules.Additionally, the presence of the fluorine and trifluoromethyl groups in the compound enhances its reactivity and stability, making it a valuable tool for the selective modification of aromatic compounds. Overall, 2-[2-Fluoro-3-(trifluoromethyl)phenyl]-4,4,5,5-tetramethyl-1,3,2-dioxaborolane plays a crucial role in modern synthetic organic chemistry by enabling the efficient and controlled formation of carbon-carbon bonds in a variety of chemical transformations.
 sales@aaronchem.com
sales@aaronchem.com