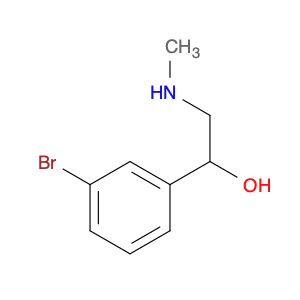
The compound 3-Bromo-α-[(methylamino)methyl]benzenemethanol, also known as $name$, is a versatile chemical reagent widely used in organic synthesis. Its unique structure with a bromine atom, methylamino group, and a benzene ring, enables it to participate in a variety of chemical reactions and transformations.One key application of $name$ in chemical synthesis is its role as a versatile building block for the preparation of various biologically active compounds. By utilizing its bromine functionality, $name$ can be easily coupled with other molecules through nucleophilic substitution reactions or palladium-catalyzed cross-coupling reactions. This enables the synthesis of complex pharmaceuticals, agrochemicals, and functional materials with high efficiency and precision.Another important aspect of using 3-Bromo-α-[(methylamino)methyl]benzenemethanol in chemical synthesis is its ability to introduce a chiral center into the molecule. The presence of the chiral center in $name$ provides an opportunity to create enantiopure compounds, which are crucial in drug discovery and development, as well as in the preparation of advanced materials with specific stereochemical properties.Furthermore, the methylamino group in $name$ can act as a directing group in various transition metal-catalyzed reactions, facilitating the selective functionalization of the benzene ring. This allows for the efficient construction of complex molecular scaffolds and the synthesis of diverse functionalized aromatic compounds with high regio- and stereo-selectivity.In summary, 3-Bromo-α-[(methylamino)methyl]benzenemethanol is a valuable reagent in chemical synthesis due to its versatility, enabling the construction of complex molecules, introduction of chirality, and selective functionalization of aromatic systems. Its applications span across medicinal chemistry, materials science, and agrochemical research, making it a crucial tool for synthetic chemists in various fields.
 sales@aaronchem.com
sales@aaronchem.com