Login |
Create New Account
 sales@aaronchem.com
sales@aaronchem.com
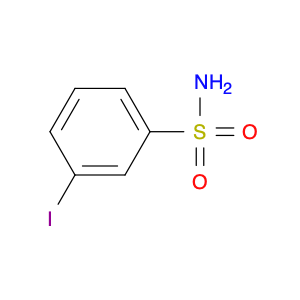
3-Iodobenzenesulfonamide
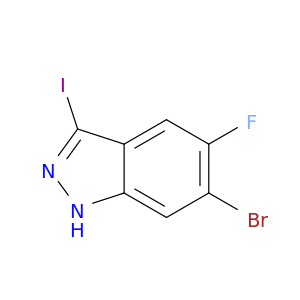
Catalog#: AR00DZMS | CAS#: 50702-39-1 | MDL#: MFCD06409088 | MF: C6H6INO2S | MW: 283.0868
| Packsize | Purity | Price | Availability | Quantity | ||
|---|---|---|---|---|---|---|
| 50mg | $211.00 | 2-3 weeks | Buy Now | Add To Cart | ||
| 100mg | $303.00 | 2-3 weeks | Buy Now | Add To Cart | ||
| 250mg | $423.00 | 2-3 weeks | Buy Now | Add To Cart | ||
| 500mg | $685.00 | 2-3 weeks | Buy Now | Add To Cart | ||
| 1g | $870.00 | 2-3 weeks | Buy Now | Add To Cart | ||
| 2.5g | $1,678.00 | 2-3 weeks | Buy Now | Add To Cart | ||
| 5g | $2,472.00 | 2-3 weeks | Buy Now | Add To Cart |
- Description
- Application
- Related Products
- Featured Products
- Safety Information
| Catalog Number | AR00DZMS |
| Chemical Name | 3-Iodobenzenesulfonamide |
| CAS Number | 50702-39-1 |
| Molecular Formula | C6H6INO2S |
| Molecular Weight | 283.0868 |
| MDL Number | MFCD06409088 |
| SMILES | Ic1cccc(c1)S(=O)(=O)N |
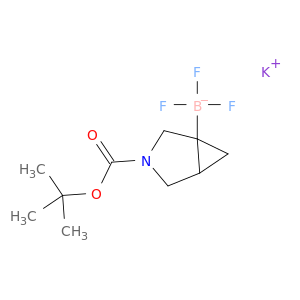
potassium {3-[(tert-butoxy)carbonyl]-3-azabicyclo[3.1.0]hexan-1-yl}trifluoroboranuide
2095504-38-2
| GHS Pictogram | N/A |
| UN# | - |
| Hazard Statements | - |
| Class | - |
| Packing Group | - |