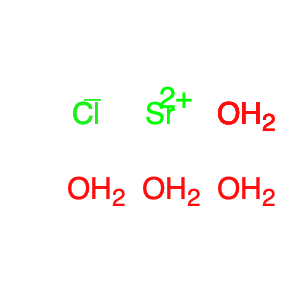
Strontium chloride hexahydrate is a versatile chemical compound commonly employed in chemical synthesis processes. Due to its unique properties, it serves as a valuable reagent in various reactions, particularly in the field of inorganic and coordination chemistry. In chemical synthesis, Strontium chloride hexahydrate is frequently utilized as a precursor for the preparation of other strontium compounds. Its solubility in water makes it a convenient source of strontium ions, which are essential for the formation of strontium-based coordination complexes. These complexes play a crucial role in the development of advanced materials with specific properties, such as luminescence or magnetic behavior. Furthermore, Strontium chloride hexahydrate can participate in metathesis reactions with other metal salts, leading to the creation of new compounds with tailored functionalities. This compound's ability to act as a Lewis acid can also facilitate certain organic transformations, making it a valuable tool in organic synthesis applications. Overall, the multifaceted role of Strontium chloride hexahydrate in chemical synthesis highlights its significance in expanding the repertoire of available reagents and enabling the creation of innovative compounds with diverse applications.
 sales@aaronchem.com
sales@aaronchem.com