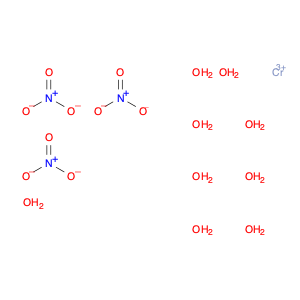
Chromium(III) nitrate nonahydrate, often referred to simply as chromium nitrate, is a versatile and powerful chemical compound used extensively in chemical synthesis applications. This inorganic compound is a key reagent in various reactions and processes due to its unique properties.One of the primary applications of chromium(III) nitrate nonahydrate is its role as an oxidizing agent in organic synthesis. It is commonly used in reactions that require oxidative conditions, such as the conversion of alcohols to carbonyl compounds or the oxidation of aromatic compounds. This compound's ability to facilitate these transformations effectively makes it a valuable tool in the synthesis of various organic compounds.In addition to its oxidizing properties, chromium nitrate also serves as a catalyst in certain reactions. Its presence can enhance the rate of a chemical reaction without being consumed in the process, making it an efficient catalyst for selective transformations in organic synthesis. This catalytic activity allows for the selective formation of specific products in complex chemical reactions.Furthermore, chromium(III) nitrate nonahydrate plays a crucial role in the preparation of coordination compounds and metal complexes. Its ability to coordinate with various ligands enables the synthesis of a wide range of coordination complexes with diverse structures and properties. These complexes have applications in areas such as catalysis, material science, and medicinal chemistry.Overall, the application of chromium(III) nitrate nonahydrate in chemical synthesis extends to its functions as an oxidizing agent, a catalyst, and a precursor for the synthesis of coordination compounds. Its versatility and effectiveness in promoting various chemical reactions make it a valuable component in the toolkit of researchers and chemists engaged in synthetic chemistry.
 sales@aaronchem.com
sales@aaronchem.com