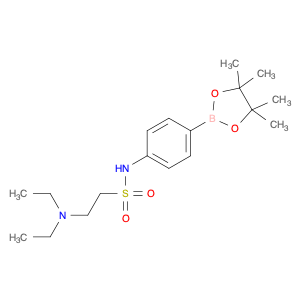
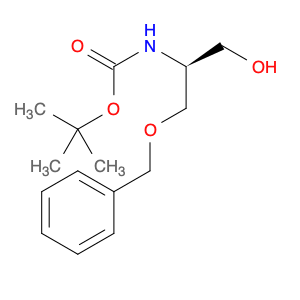
2-(Diethylamino)-N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) phenyl] ethanesulfonamide is a versatile compound widely used in chemical synthesis for its unique reactivity and structural properties. This compound serves as a valuable building block in organic chemistry, particularly in the field of medicinal and pharmaceutical chemistry.In synthetic chemistry, this compound is utilized as a key reagent in the formation of complex molecular structures due to its ability to undergo various chemical transformations. One of its primary applications is in the Suzuki-Miyaura cross-coupling reaction, a powerful method for forming carbon-carbon bonds. By reacting with aryl halides or pseudohalides in the presence of a palladium catalyst, 2-(Diethylamino)-N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) phenyl] ethanesulfonamide facilitates the coupling of two different organic molecules, enabling the synthesis of biaryl compounds with high efficiency.Moreover, this compound's sulfonamide moiety provides additional functionality for further derivatization, allowing for the introduction of various functional groups to tailor the compound's properties for specific applications. Its electron-rich boron-containing aromatic ring also confers unique reactivity, making it a valuable tool for the construction of diverse molecular architectures in chemical synthesis.Overall, 2-(Diethylamino)-N-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) phenyl] ethanesulfonamide plays a crucial role in modern organic synthesis by enabling the synthesis of complex organic molecules with diverse functionalities, making it a versatile and indispensable compound for chemical research and development.
 sales@aaronchem.com
sales@aaronchem.com