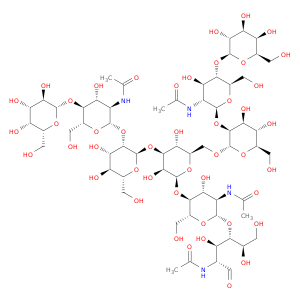
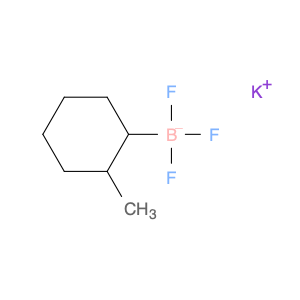
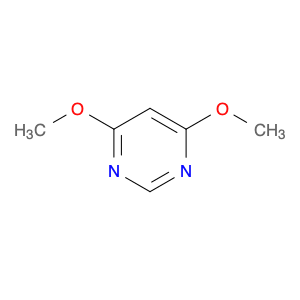
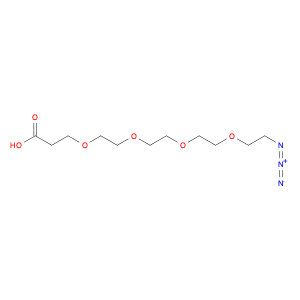
G2 Glycan, a versatile compound in chemical synthesis, is a crucial component used in various reactions to enhance the efficiency and specificity of processes. Its unique structure and properties make it an invaluable tool for researchers and chemists looking to achieve precise and controlled results in their synthetic endeavors.One of the key applications of G2 Glycan in chemical synthesis is its ability to serve as a chiral ligand in asymmetric catalysis. By incorporating G2 Glycan into catalytic systems, chemists can promote stereoselective reactions, leading to the production of enantiomerically pure compounds. This is particularly important in the pharmaceutical industry, where the chirality of molecules can significantly impact their biological activity and interactions within the body.Furthermore, G2 Glycan can also be utilized as a protecting group in organic synthesis. Its selective reactivity towards specific functional groups allows chemists to mask sensitive functionalities temporarily, enabling them to carry out complex transformations without unwanted side reactions. This protective role of G2 Glycan is especially helpful in multi-step syntheses where the preservation of certain moieties is crucial for the overall success of the process.In addition to its role in asymmetric catalysis and protection strategies, G2 Glycan can act as a glycosylation reagent in carbohydrate chemistry. Its ability to participate in glycosylation reactions under mild conditions makes it a valuable tool for the synthesis of oligosaccharides and glycoconjugates. By facilitating the formation of glycosidic bonds, G2 Glycan opens up avenues for the creation of structurally diverse and biologically relevant carbohydrate derivatives.Overall, the versatile applications of G2 Glycan in chemical synthesis highlight its significance as a key reagent for achieving complex molecular transformations with precision and control. Chemists across various fields rely on the unique properties of G2 Glycan to advance their research and development efforts, making it an indispensable tool in the realm of synthetic chemistry.
 sales@aaronchem.com
sales@aaronchem.com