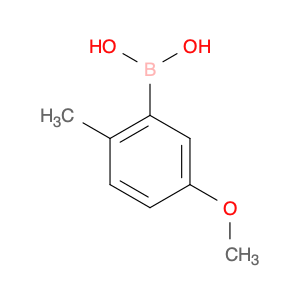
5-Methoxy-2-methylphenylboronic acid, also known as 5-Methoxy-2-methylbenzeneboronic acid, is a versatile compound widely used in various chemical syntheses. Its primary application lies in the field of organic chemistry, particularly in the area of Suzuki-Miyaura cross-coupling reactions. This boronic acid derivative serves as an essential building block in the formation of carbon-carbon bonds, playing a crucial role in the construction of complex organic molecules.In organic synthesis, the Suzuki-Miyaura cross-coupling reaction is a valuable tool for creating new carbon-carbon bonds between an aryl or vinyl boronic acid and an organic halide or pseudohalide. 5-Methoxy-2-methylphenylboronic acid acts as a key component in this reaction, serving as a nucleophilic partner that undergoes transmetallation with a palladium catalyst to form an intermediate arylpalladium complex. Subsequent reductive elimination leads to the desired coupling product, enabling chemists to access a wide range of biaryl compounds, natural products, and pharmaceutical intermediates.By harnessing the unique reactivity of 5-Methoxy-2-methylphenylboronic acid in Suzuki-Miyaura cross-coupling reactions, chemists can efficiently and selectively synthesize diverse organic molecules with high control over regioselectivity and functional group compatibility. This compound's versatility and utility make it a valuable tool in the arsenal of synthetic chemists striving to design and access novel compounds for applications in drug discovery, materials science, and other fields.
 sales@aaronchem.com
sales@aaronchem.com