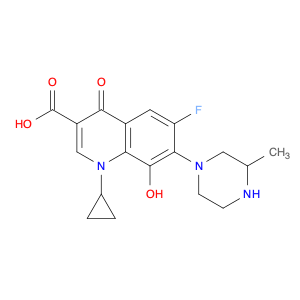
The compound 1-Cyclopropyl-6-fluoro-8-hydroxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid, often referred to as $name$, plays a crucial role in chemical synthesis as a versatile building block. Its unique structure contains a quinolone backbone with functional groups that can participate in various chemical reactions.In organic synthesis, $name$ can be utilized as a key intermediate in the preparation of advanced pharmaceutical compounds. Its cyclopropyl and fluoro substitutions confer specific reactivity patterns, allowing for targeted modifications to be made during the synthesis process. The hydroxy and carboxylic acid groups provide opportunities for further derivatization, enabling the introduction of additional functionalities for fine-tuning the properties of the final product.Additionally, the presence of the 3-methylpiperazin-1-yl moiety in $name$ enhances its pharmacological potential, making it a valuable precursor for the development of bioactive molecules. By strategically incorporating this structural motif into the target compound, researchers can explore its implications on biological activity and pharmacokinetic properties.Overall, the strategic placement of functional groups in 1-Cyclopropyl-6-fluoro-8-hydroxy-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid offers chemists a platform for designing and synthesizing novel compounds with tailored properties for various applications in medicinal chemistry and drug discovery.
 sales@aaronchem.com
sales@aaronchem.com