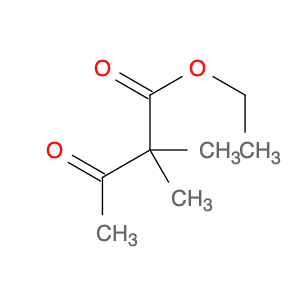
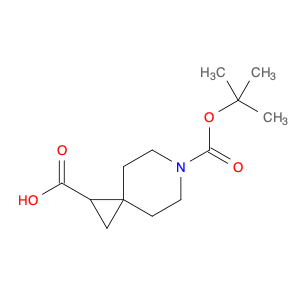
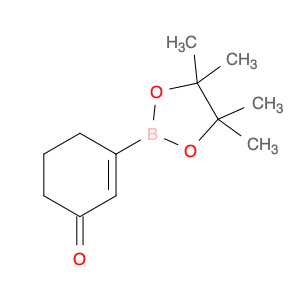
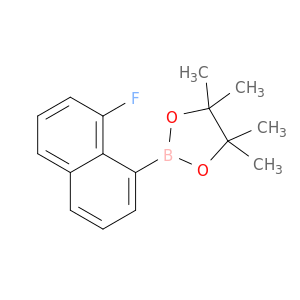
Ethyl 2,2-dimethyl-3-oxobutanoate, also known as Diethyl acetylmalonate, is a versatile compound widely used in organic synthesis. This compound serves as a valuable building block in the preparation of various organic compounds due to its unique structural characteristics.In chemical synthesis, Ethyl 2,2-dimethyl-3-oxobutanoate is commonly employed as a key intermediate in the synthesis of complex molecules such as pharmaceuticals, agrochemicals, and specialty chemicals. Its enolizable nature makes it a suitable substrate for a variety of reactions, including alkylation, acylation, and condensation reactions.One prominent application of Ethyl 2,2-dimethyl-3-oxobutanoate is in the synthesis of pyrazoles, which are important heterocyclic compounds with diverse biological activities. By reacting Ethyl 2,2-dimethyl-3-oxobutanoate with hydrazine derivatives, pyrazole derivatives can be efficiently synthesized through cyclization reactions.Additionally, Ethyl 2,2-dimethyl-3-oxobutanoate can undergo Michael addition reactions with various nucleophiles, enabling the introduction of functional groups at the β-carbon position. This reactivity is particularly useful in the construction of carbon-carbon bonds and the creation of structural diversity in organic molecules.Overall, Ethyl 2,2-dimethyl-3-oxobutanoate plays a crucial role in chemical synthesis by serving as a key intermediate for the preparation of complex organic compounds with diverse applications in the fields of pharmaceuticals, agrochemicals, and materials science.
 sales@aaronchem.com
sales@aaronchem.com