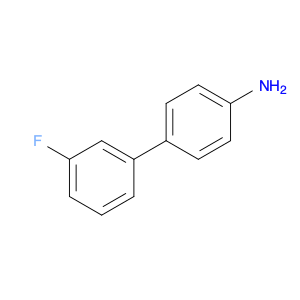
The 3'-Fluoro-[1,1'-biphenyl]-4-amine is a versatile compound widely used in chemical synthesis due to its valuable properties and reactivity patterns. This compound acts as a vital building block in organic synthesis reactions, contributing to the creation of various pharmaceuticals, agrochemicals, and materials.Firstly, the 3'-Fluoro-[1,1'-biphenyl]-4-amine can undergo effective cross-coupling reactions with aryl halides, enabling the formation of complex molecular structures. These reactions are crucial in the design and synthesis of new drugs and agrochemicals, as well as in the production of advanced materials with tailored properties.Additionally, this compound exhibits unique steric and electronic properties that make it an excellent substrate for Suzuki-Miyaura coupling reactions. By utilizing this compound in such reactions, chemists can efficiently construct biaryl compounds, which are key structural motifs found in a wide range of bioactive molecules and pharmaceuticals.Furthermore, the presence of the fluoro group in the 3' position of the biphenyl ring offers additional opportunities for selective functionalization via various chemical transformations. This allows for the fine-tuning of the compound's properties and facilitates the synthesis of diverse molecular scaffolds for drug discovery and materials science applications.In summary, the 3'-Fluoro-[1,1'-biphenyl]-4-amine plays a crucial role in chemical synthesis processes by enabling the construction of intricate molecular architectures essential for the development of new drugs, agrochemicals, and advanced materials. Its versatile reactivity and unique structural features make it a valuable tool for chemists seeking to access a diverse array of functionalized compounds for various applications.
 sales@aaronchem.com
sales@aaronchem.com