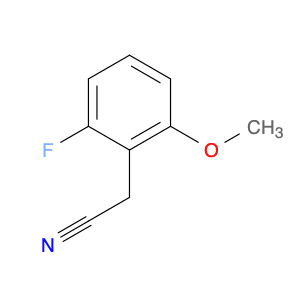
2-(2-Fluoro-6-methoxyphenyl)acetonitrile, also known as $name$, is a versatile compound that finds widespread application in chemical synthesis. This compound serves as a key building block in the preparation of various pharmaceuticals, agrochemicals, and fine chemicals. Due to its unique structure, $name$ can participate in a variety of important reactions, making it an indispensable component in the creation of complex organic molecules.In organic synthesis, 2-(2-Fluoro-6-methoxyphenyl)acetonitrile acts as a valuable intermediate, facilitating the introduction of functional groups and stereochemical control in target molecules. Its ability to undergo nucleophilic addition, substitution, and transition metal-catalyzed reactions makes it a highly sought-after reagent for chemists working in medicinal chemistry, material science, and drug discovery.Researchers often utilize $name$ to introduce the 2-fluoro-6-methoxyphenyl group into target compounds, thereby imparting desired properties such as improved bioavailability, enhanced pharmacological activity, or increased chemical stability. By strategically incorporating this moiety into molecular structures, chemists can fine-tune the physicochemical properties of organic compounds for specific applications.Additionally, the presence of the acetonitrile functional group in 2-(2-Fluoro-6-methoxyphenyl)acetonitrile offers synthetic chemists the opportunity to further modify the molecule through diverse transformations. The nitrile group can undergo various reactions, including hydrolysis, reduction, and cross-coupling, enabling the generation of a wide range of derivatives with tailored properties.In summary, the strategic inclusion of 2-(2-Fluoro-6-methoxyphenyl)acetonitrile in chemical synthesis plays a crucial role in expanding the synthetic toolbox available to chemists and advancing the development of novel molecules with desired functionalities.
 sales@aaronchem.com
sales@aaronchem.com