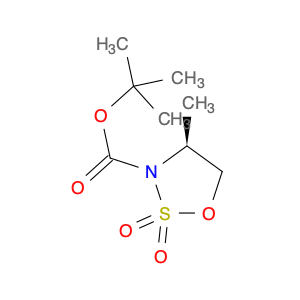
(S)-tert-Butyl 4-methyl-1,2,3-oxathiazolidine-3-carboxylate 2,2-dioxide is a versatile compound commonly used in chemical synthesis processes. Its unique chemical structure makes it an essential building block in the production of various pharmaceuticals, agrochemicals, and fine chemicals.This compound is known for its chirality, which allows it to participate in asymmetric synthesis, leading to the formation of enantiomerically pure products. Its presence in a reaction mixture can selectively influence the formation of desired enantiomers, making it a valuable tool for the synthesis of optically active compounds.In addition, (S)-tert-Butyl 4-methyl-1,2,3-oxathiazolidine-3-carboxylate 2,2-dioxide can serve as a protecting group for amines, alcohols, and other functional groups during multi-step synthesis processes. By temporarily masking certain reactive sites, this compound helps to control the sequence of chemical transformations, leading to the efficient and selective formation of complex molecules.Furthermore, this compound's stability and compatibility with a wide range of solvents and reagents make it a preferred choice in various synthetic methodologies, including organocatalysis, transition-metal catalysis, and radical reactions. Its ease of handling and high reactivity under mild conditions contribute to its widespread use in the preparation of novel compounds with diverse applications.Overall, (S)-tert-Butyl 4-methyl-1,2,3-oxathiazolidine-3-carboxylate 2,2-dioxide plays a crucial role in modern chemical synthesis strategies, enabling chemists to access structurally complex and biologically active molecules with high efficiency and precision.
 sales@aaronchem.com
sales@aaronchem.com