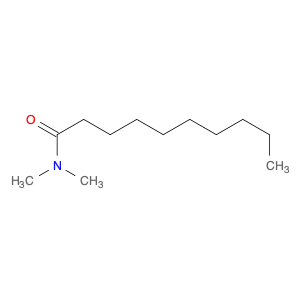
N,N-Dimethyldecanamide, also known as DMDA, is a versatile compound widely used in chemical synthesis for its unique properties. As a highly polar solvent, DMDA is valued for its ability to dissolve a wide range of organic and inorganic substances, making it a valuable tool in organic synthesis. It is commonly utilized as a reaction medium in various chemical transformations, such as nucleophilic substitutions, Grignard reactions, and Suzuki couplings.DMDA's high boiling point and low volatility make it particularly suitable for reactions requiring elevated temperatures or prolonged reaction times. Additionally, its low viscosity and high solubility in both polar and nonpolar solvents allow for efficient mixing and dissolution of reactants, leading to improved reaction yields and purity of products.Furthermore, DMDA serves as an effective reagent for a variety of transformations, including amide bond formation, amidation reactions, and as a protecting group in peptide synthesis. Its compatibility with a wide range of functional groups and its stability under acidic and basic conditions make it a valuable asset in complex chemical syntheses.Overall, N,N-Dimethyldecanamide plays a crucial role in chemical synthesis by providing a versatile and reliable solvent system for a diverse array of reactions, enabling chemists to efficiently and effectively carry out complex organic transformations to access a wide range of valuable compounds.
 sales@aaronchem.com
sales@aaronchem.com